Influenza (FLU) Update for Week Ending 4-27-19
For week #16 & 17 (ending 4-20 & 4-27-19) the CDC reported that Influenza (Flu) activity, which includes diagnosed flu as well as ILI (Influenza-Like-Illness), continued to decline during Weeks #16 and 17, as this year’s Flu Season finally showed a decrease in almost all the flu markers. Only a few states (3) had widespread flu activity and ILI is now under the national baseline of 2.2% for the second week in a row. All Regions reported “Normal” activity with ILI less than each of their region-specific baselines. The percentage of respiratory specimens testing positive for flu decreased to 5.4%% from 8.1% last week, both much lower than the double digits seen since November. And, although the number of hospitalizations from flu continued to increase, it was a small. Adult mortality from pneumonia and influenza was again below the epidemic threshold. Weeks #16 & 17 added to pediatric morality from flu with five (5) deaths each week. The CDC expects flu and ILI activity to continue to wane but sporadic activity may occur for a few more weeks. Although it is a record long flu season, it doesn’t come close to the severity of the 2017/18 Flu Season.
In the samples tested, Influenza A viruses still held the largest percentage as it has since late February, however Influenza B has increased the past few weeks, as is expected in Spring. As it has been the past few weeks, Influenza A(H3) was more prevalent nationally and dominant in all the Regions. Overall, Influenzas A(H1N1), A(H3N2), and Influenza B viruses were co-circulating, with Influenza B cases increasing, a sign typically seen in Spring. A(H3N2) viruses typically cause more severe illness in older adults and the flu vaccine often covers H3N2 less well in this population.
The majority of the flu viruses were genetically similar to the 2018/19 Flu Vaccine, but the majority of Influenza A(H3N2) viruses are antigenically different from the H3N2 reference virus used in the 2018/19 North American Hemisphere Flu Vaccine. To allow for this, changes to the 2019/20 North American Hemisphere Vaccine have been recommended and adopted.
The CDC published its 2018/19 Flu Season Preliminary Burden Estimates, & from October 1, 2018 through April 27, 2019, there have been an estimated 37.2 – 4.7 million flu illnesses, 17.2 – 20 million medical visits for flu, 524,000 – 637,000 flu-related hospitalizations, and 36,100 – 59,600 flu deaths.
This link provides info on the EPI prediction: https://www.cdc.gov/fslu/weekly/flusight/index.html
FirstWatch RIN (Regional Influenza Network): RIN Alerts for Weeks #16 & 17 still showed a sizable number with no waning so far.
For the most recently reported week, ending April 27, 2019, the CDC reported:
Influenza-like illness (ILI) visits to clinics & other non-hospital facilities decreased to 1.8% (l. w. 2.1%), below the national baseline of 2.2% for both weeks. All regions reported levels below their region-specific baselines, with a range of 0.9% to 2.8%.
Flu cases, documented by positive flu tests of respiratory specimens, were reported as Widespread in only three (3) states. Clinical lab testing for influenza was positive for 5.4% of specimens, compared to 8.1% last week, with a range of 5.6% (Region 3, Mid-Atlantic) to 15.3% (Region 1, Northeast). Only three (3) regions were in the double digits.
Influenza A remained the dominant flu for 73.1% of the flu tests reported (last week 76.2%), but the rest were a rising number of Influenza B at 26.9% (23.8% l.w.). The H3N2 subtype remained the dominant Influenza A virus at 67% with A(H1N1)pdm09 viruses at 33%. Of the Influenza B results, Yamagata was at 34.4% and Victoria at 69.9% (sic). Typically, Influenza B viruses occur more toward Spring and cause less severe illness. A(H3N2) viruses are known to cause increased severity and be less covered by the flu vaccine. This pattern is mirrored in much of the world.
More than 99% of the flu viruses tested were found to be sensitive to the antivirals oseltamivir, zanamivir (100%), and peramivir (Tamiflu, Relenza, and Rapivab, respectively).
The CDC recommends treatment with antivirals, as early as possible, for those with confirmed or suspected flu with severe, complicated, or progressive disease, those who are hospitalized, or at high risk for complications of flu. See this link for a list of those at risk for complications from flu: https://www.cdc.gov/flu/about/disease/high_risk.htm
The CDC provides an interactive U.S. map that will link to each state’s public health authorities. ILI and Flu information and processes, as well as other diseases and public health topics. This site includes a tremendous amount of information at the State and even Local level. Find it at this site: https://www.cdc.gov/flu/weekly/usmap.htm
For Influenza-Like Illness:
High ILI Activity: Puerto Rico
Moderate ILI Activity: None
Low Activity: (4 states): Alaska, Arizona, Kentucky, and Louisiana
Minimal Activity: (New York City, Washington D.C. & 46 states): Alabama, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin and Wyoming
Insufficient Data: the U.S. Virgin Islands
For Flu (positive flu tests):
Widespread Activity: (3 states): Connecticut, Massachusetts, and New York
Regional Activity (Puerto Rico & 7 states): Arizona, Georgia, Maine, Nevada, Ohio, Rhode Island, and Utah
Local Activity: (18 states): Delaware, Florida, Kentucky, Louisiana, Maryland, Michigan, Minnesota, Mississippi, Montana, New Hampshire, New Mexico, North Carolina, North Dakota, Pennsylvania, Tennessee, Washington, West Virginia, and Wisconsin
Sporadic Activity: (Washington D.C., U.S. Virgin Islands & 22 states): Alabama, Alaska, Arkansas, California, Colorado, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Missouri, Nebraska, New Jersey, Oklahoma, Oregon, South Carolina, South Dakota, Texas, Vermont, Virginia, and Wyoming
Guam and did not report
Other Data:
The hospitalization rate from Flu continued to increase to 64.7 per 100,000 (last week 64.2/100,000), but is leveling off and increasing at a much slower pace. Older adults (age > 65 years) had the highest hospitalization rate at 216.6 per 100,000 (l.w. 214.1/ ); adults (age 50-64 years) were at 80 per 100,000 (l.w. 79.7/ ); and children (ages 0-4) had 74 per 100,000 (last week 73.1/ ).
As of 5/2/19, the death rate for pneumonia & influenza in adults was at 6.3% and below the epidemic threshold of 6.9% for week #16. Note: the epidemic threshold number may change from week to week. Death reports often aren’t reported for data purposes the same week and are typically reported by the CDC a week behind.
There was a total of 10 pediatric deaths, attributed to flu, reported this week; five (5) each for Weeks #16 & 17. This has resulted in a total of 101 deaths, so far, for this Flu Season.
Flu in Canada, Europe & the World:
Canada:
According to the Public Health Agency of Canada (PHAC), for Week #17, ending 4/27/19, flu activity is declining, including the second smaller wave in which A(H3N2) dominated. However, A(H3N2) and Influenza B still circulated throughout many Regions of the country. The PHAC also reported that Influenza A(H3N2) cases have increased since the middle of Jan and represented 82% of the Influenza A subtyping this week, compared to 89% for last week, although A(H1N1)pdm09 was still the dominant type for this Flu Season as a whole. Meanwhile, very little Influenza B has been identified this season when compared to other seasons.
Widespread Activity in 0 Regions
Localized Activity in 13 Regions: Ont. (6), Que (3), N.B. (1), N.S. (1) & N.L. (2)
Sporadic Activity in 27 Regions: B.C. (4), Sask. (3), Man. (4), Que (3), N.S. (3), N.B. (5), P.E.I. (1) N.L. (2), Y.T. (1), & N.W.T. (1)
No Activity Reported in 4 Regions in 4 different provinces
For more specific information see:
On flu activity: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fluwatch/2018-2019/week17-april-21-april-27-2019.html
Canadian Flu Information: https://www.canada.ca/en/public-health/services/diseases/flu-influenza.html
General Page for Canadian Flu Watch Surveillance with links to different components:
https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance.html
About the Canadian Influenza Activity Surveillance System:
https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance/about-fluwatch.html
Europe:
According to the European Center for Disease Prevention & Control (ECDC), for Week #17 (Apr 22 – 28, 2019), of those countries reporting on geographic spread of influenza activity, few of them reported flu detections and, of those that did, the number found was low. All the countries reporting ILI or ARI (acute respiratory illness) reported levels at baseline, suggesting that the influenza season for Europe is coming to an end. The samples taken from those with ILI or ARI by sentinel primary healthcare sites, decreased to 16% positive for flu viruses and this rate was mostly because of reporting from one (1) country. Those reported with severe acute respiratory infection (SARI) that were tested for flu viruses, had a positive result of 3.5%; all were Influenza A. Overall, Influenza A viruses dominated. Data from 20 reporting Member States and areas that reported to the EuroMOMO project, indicated that excess mortality from earlier weeks had returned to expected levels for this time.
For more information see: https://flunewseurope.org/
World: The World Health Organization (WHO) provides info on Influenza in Member Countries here: https://www.who.int/influenza/surveillance_monitoring/en/
First Responder Specific Information
There are many websites that may be helpful in planning and managing seasonal flu within First Responder organizations. A few of those websites are included here:
NIOSH on Flu for Employers/Employees:
https://www.cdc.gov/niosh/topics/flu/
Protection from Flu:
https://www.cdc.gov/flu/protect/habits/index.htm
Weekly Flu Map:
https://www.cdc.gov/flu/weekly/usmap.htm
World Map Showing Flu & Other Infectious Diseases:
https://www.healthmap.org/en/
Other Actions First Responders Should Consider
- First Responders should be vaccinated for Flu each season to prevent getting flu themselves, taking it home to family members, or transmitting it to patients in their care. Family members and patients may be at increased risk of complications from flu.
- Perform proper hand hygiene including frequent handwashing and the use of hand sanitizers in general, and particularly when providing patient care or after touching surfaces.
- Masks (N95 or N100) should be used in the presence of patients with cough and/or fever; preferably before being within 6 feet of the patient. This becomes even more important if droplet producing procedures are being performed (i.e. suctioning, nebulizer treatments, BVM, intubation).
- Care should be taken to avoid touching your own face and mucous membranes (eyes, mouth, nose) since the flu virus is frequently found on surfaces such as door knobs, writing & recording tools (pens and tablets), cot and equipment handles, phones, light switches, as well as clothing, bed clothes, etc.
- Report signs/symptoms of flu to your physician or other appropriate provider for early assessment and care. Alert your employer per policy.
- Cough and sneeze into your sleeve, if a tissue is not available, and not onto your hands. Watch this Youtube video for a humorous but educational approach on the subject. https://www.youtube.com/watch?v=CtnEwvUWDo0
- Stay away from others if you are sick.
- Be aware of your exposure risk and history to prevent exposing others. Take extra precautions or avoid those with immunocompromise, when possible, if you have a known or likely exposure.
- Antivirals may be indicated for the treatment of flu, particularly for those in high risk groups, those who are hospitalized or have severe, complicated or progressing flu. Those that present with 48 hours of the onset of symptoms may also be given antivirals, based on PCP judgement but make sure the practitioner is aware of their First Responder Role. See https://www.cdc.gov/flu/antivirals/whatyoushould.htm
And, for consideration when looking at yourself, your family and friends, or your patients, consider the following information regarding complications of flu:
Flu is much more worrisome for the very young and the elderly, as well as those who fit into one of the high risk categories see this link for the list: https://www.cdc.gov/flu/about/disease/high_risk.htm . Signs of ILI/Flu in this group requires careful assessment to rule out complications and these groups are much more likely to need medical oversight to assure adequate care. Young children and those over 65 are typically at greater risk for complications, hospitalization, and even death.
Consideration should be given to perhaps monitoring these groups more closely, with inclination for more comprehensive assessment and transport for further evaluation, when presented with possible flu and any signs of complications.
Complications of flu, sometimes requiring hospitalization and even leading to death, tend to occur after the person has begun to get better from the flu and then appears to relapse. EMS personnel may want to look more closely at those patients when the call is not about the initial signs and symptoms of flu, but about increasing or different signs that have appeared, often from five days to two weeks after the initial flu symptoms began.
A study was published by the Institute for Clinical Evaluative Sciences in NEJM (New England Journal of Medicine). See details below:
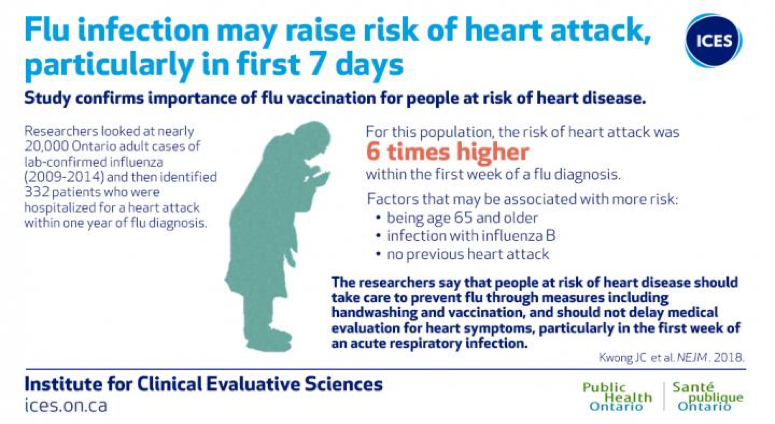
Image courtesy of ICES/PHO
“The researchers add that patients should not delay medical evaluation for heart symptoms particularly within the first week of an acute respiratory infection.” (Lisa Schnirring, News Editor: CIDRAP News ;Jan 25, 2018)
For more information on Influenza and the Heart Attack Study, please see the link below.
https://www.eurekalert.org/pub_releases/2018-01/pho-rcl011818.php

